FDA Cancer CAR T Warning A Critical Look
FDA Cancer CAR T warning is a significant development in cancer treatment, prompting crucial questions about safety and efficacy. This warning affects certain cancer types and raises concerns about potential side effects of CAR T-cell therapies. Understanding the details behind the FDA’s actions and the broader implications for cancer treatment is essential for patients, healthcare providers, and the public.
The FDA’s warning underscores the need for thorough safety monitoring and ongoing research to ensure the long-term success and safety of CAR T-cell therapy. This complex issue requires careful consideration of potential risks and benefits for individual patients.
FDA Warning Context
The FDA recently issued a crucial warning regarding CAR T-cell therapies, highlighting potential serious side effects and prompting important considerations for patients and healthcare providers. This warning underscores the need for vigilance and careful monitoring in the use of these innovative cancer treatments.
Summary of the FDA Warning, Fda cancer car t warning
The FDA’s warning centers on the potential for severe and sometimes life-threatening side effects in patients undergoing CAR T-cell therapy. While CAR T-cell therapies have shown remarkable success in treating certain cancers, the FDA emphasized the need for heightened awareness of the potential risks. The warning specifically addresses the importance of careful monitoring for these side effects and the need for proactive management strategies.
Types of Cancer Affected
The FDA’s warning encompasses several types of blood cancers, including lymphoma and leukemia. The warning particularly focuses on the use of CAR T-cell therapies in treating these malignancies.
Potential Side Effects
The FDA highlighted a range of potential side effects, emphasizing that these therapies can trigger a variety of severe inflammatory responses in the body. These responses can lead to significant complications, including cytokine release syndrome (CRS), neurotoxicity, and immune effector cell-associated neurotoxicity syndrome (ICANS). Furthermore, the FDA noted the potential for long-term effects, requiring ongoing monitoring of patients even after treatment.
FDA’s Rationale for the Warning
The FDA’s rationale for issuing this warning stems from the need to ensure the safe and effective use of CAR T-cell therapies. The warning acknowledges the therapeutic potential while emphasizing the need for vigilance and proactive management strategies. This approach aims to balance the benefits of these therapies with the necessity of mitigating their potential risks.
Impact on Patients and Healthcare Providers
This warning will impact patients by increasing awareness of the potential risks associated with CAR T-cell therapy. Healthcare providers will need to be more vigilant in monitoring patients for side effects and developing tailored management strategies. The warning will also likely lead to more comprehensive pre-treatment discussions with patients, emphasizing the potential benefits and risks.
Table of CAR T-cell Therapies
| Type of CAR T-cell Therapy | Associated Cancer | Reported Side Effects (FDA Warning) | Additional Notes |
|---|---|---|---|
| Chimeric Antigen Receptor (CAR) T-cell Therapy targeting CD19 | B-cell lymphomas and leukemias | Cytokine Release Syndrome (CRS), Neurotoxicity, Immune effector cell-associated neurotoxicity syndrome (ICANS) | This type of therapy is commonly used for certain types of blood cancers. |
| CAR T-cell Therapy targeting CD22 | B-cell lymphomas and leukemias | Cytokine Release Syndrome (CRS), Neurotoxicity, Immune effector cell-associated neurotoxicity syndrome (ICANS) | This therapy also targets B-cell malignancies. |
| CAR T-cell Therapy targeting other antigens | Various hematological malignancies and potentially solid tumors | Cytokine Release Syndrome (CRS), Neurotoxicity, Immune effector cell-associated neurotoxicity syndrome (ICANS), and others specific to the target antigen. | Emerging CAR T-cell therapies are targeting a wider range of cancers and may present different side effects. |
Historical Background of CAR T-cell Therapy
CAR T-cell therapy, a revolutionary approach to cancer treatment, has rapidly evolved from a theoretical concept to a widely used clinical modality. This innovative treatment harnesses the power of a patient’s own immune system to target and eliminate cancer cells. Its development has been marked by significant breakthroughs and ongoing refinement, leading to improvements in both efficacy and safety.The journey of CAR T-cell therapy began with the initial understanding of T-cell receptors (TCRs) and their ability to recognize and destroy foreign invaders.
Early research focused on engineering T-cells to recognize specific cancer antigens, paving the way for the development of chimeric antigen receptors (CARs). This intricate process involves genetically modifying T-cells to express CARs, which are designed to bind to specific proteins on the surface of cancer cells. This targeted approach has shown promise in treating various cancers, particularly hematological malignancies.
Early Concepts and Milestones
Early attempts to redirect T-cells for cancer therapy focused on understanding the basic mechanisms of T-cell activation and targeting. Key breakthroughs involved the identification of crucial proteins on cancer cells that could be specifically recognized and targeted. The development of methods to isolate, modify, and re-infuse T-cells into patients was crucial in the initial stages. This marked a significant step toward transforming laboratory concepts into practical clinical applications.
Generations of CAR T-cell Therapy
The evolution of CAR T-cell therapy has been characterized by different generations of CAR constructs, each aiming to improve efficacy and safety. The progression reflects the continuous refinement of the technology.
The FDA’s recent warning about potential side effects of CAR T-cell therapy for cancer is definitely concerning. While this innovative treatment shows promise, understanding these risks is crucial. It’s interesting to consider how naming conventions for newborns might differ across cultures, especially given the increasing global reach of medical advancements like CAR T-cell therapy. For instance, exploring the various traditions surrounding naming a child, like apellido bebe madre padre , offers a fascinating look at how different societies approach the new life.
Ultimately, the FDA’s warnings about CAR T-cell therapies remain a critical discussion point in the medical community.
| Generation | CAR Structure | Efficacy Improvements | Safety Profile Improvements |
|---|---|---|---|
| First Generation | Simple single-chain variable fragment (scFv) | Limited efficacy, variable response rates. | Relatively high risk of cytokine release syndrome (CRS) and neurotoxicity. |
| Second Generation | Improved scFv, co-stimulatory domains (e.g., CD28, 4-1BB) | Enhanced efficacy, higher response rates in some cancers. | Lower risk of CRS and neurotoxicity compared to first generation. Still potential for severe side effects. |
| Third Generation | Multi-specific CARs, optimized co-stimulatory domains, T-cell engineering strategies, enhanced safety mechanisms. | Improved efficacy in a wider range of cancers. | Further reduced risk of CRS and neurotoxicity. Still potential for side effects, though manageable in many cases. |
Overall Success Rate and Cancer Types
The success rate of CAR T-cell therapy varies significantly depending on the specific cancer type and patient characteristics. In some hematological malignancies, such as acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL), CAR T-cell therapy has demonstrated remarkable success, achieving remission rates in a substantial portion of patients. However, the success rate in solid tumors remains more challenging and is actively being investigated.
The complexity of solid tumors and their heterogeneity poses a significant hurdle in achieving consistent responses. Ongoing research focuses on developing strategies to overcome these limitations, including targeting multiple antigens, enhancing T-cell infiltration into solid tumors, and improving delivery methods.
Potential Implications for Cancer Treatment
The recent FDA warning regarding certain CAR T-cell therapies underscores the complexities of innovative cancer treatments. While CAR T-cell therapy holds immense promise, the potential for severe side effects necessitates careful consideration and proactive measures. This necessitates a critical look at alternative treatment options, research adjustments, and potential modifications in patient selection.
Alternative Treatments for Impacted Patients
The FDA warning necessitates exploring alternative treatment strategies for patients whose CAR T-cell therapy is affected. These may include traditional chemotherapy regimens, targeted therapies, immunotherapies like checkpoint inhibitors, or radiation therapy. The choice of alternative depends heavily on the specific cancer type, stage of the disease, and the patient’s overall health. In some cases, a combination of therapies might be the most effective approach.
For instance, a patient whose CAR T-cell therapy is deemed unsuitable might benefit from a combination of targeted therapies and chemotherapy to achieve disease control. Early intervention and collaboration between oncologists and other healthcare providers are crucial to ensuring optimal outcomes.
Implications for Clinical Trials and Research
The FDA warning signals a need for rigorous evaluation of CAR T-cell therapy protocols in clinical trials. Trials must incorporate enhanced monitoring of patients, especially focusing on the long-term effects of the therapy. Safety data collection needs to be more comprehensive, and prospective studies should explore potential risk factors for adverse events. This shift in focus toward comprehensive safety data collection is vital to refine treatment strategies and avoid recurrence of the issues highlighted in the FDA warning.
This includes meticulous tracking of adverse events, particularly those that were previously underreported or poorly understood.
The FDA’s recent warning about potential risks associated with CAR T-cell therapy for cancer treatment has got me thinking. It’s a sobering reminder of the complexities of modern medicine. While the therapy shows promise, these potential side effects are a real concern. Interestingly, the remarkable career of Adrian Beltre, a Texas Rangers legend and future Hall of Famer, reminds us of how we celebrate human achievements in other fields.
Adrian Beltre hall of fame Texas Rangers highlights the dedication and resilience it takes to succeed. Ultimately, the FDA’s cancer CAR T-cell warning underscores the ongoing need for careful research and monitoring in this rapidly evolving medical landscape.
Impact on Future Development of CAR T-cell Therapies
The FDA warning will undoubtedly influence future CAR T-cell therapy development. Researchers will likely focus on improving the safety profiles of these therapies, potentially through engineering safer cell receptors, optimizing dosage regimens, or developing strategies to mitigate or prevent adverse events. For example, investigators might explore novel ways to engineer CAR T-cells to target specific cancer cells more precisely, thereby reducing off-target effects on healthy cells.
The FDA’s recent warning about potential risks with CAR T-cell cancer therapies is definitely a concern. It’s a complex issue, but it’s interesting to see how the legal system is working in parallel, such as in the case of Thailand’s Pita Limjaroenrat, who recently won a key legal battle. thailand pita wins case This suggests that navigating complex legal and medical landscapes requires careful consideration and a balanced approach, particularly when it comes to potentially life-saving treatments like CAR T-cell therapies.
This approach aims to minimize the severe side effects while maximizing therapeutic efficacy.
Potential Adjustments in Patient Selection Criteria
The FDA warning necessitates a reevaluation of patient selection criteria for CAR T-cell therapy. Patients undergoing this treatment will likely require more thorough assessments of their overall health, including underlying conditions and genetic predispositions that might increase the risk of adverse events. Detailed pre-treatment assessments will become more crucial, incorporating biomarkers and predictive models to identify patients who are likely to benefit most from the treatment while minimizing potential risks.
This includes assessing the patient’s capacity to manage the potential side effects and access appropriate support systems.
The FDA’s recent cancer CAR T-cell therapy warning is definitely a serious development. It’s prompting a lot of discussion about the potential risks of these innovative treatments. Meanwhile, the news about stars Harley Johnston, Oettinger, and Benn is also making headlines, raising questions about celebrity influence and public perception. The FDA’s concerns about CAR T-cell therapies, however, remain a critical issue that needs ongoing research and monitoring.
stars harley johnston oettinger benn are making news for other reasons, but the FDA’s safety concerns are important for all of us.
Comparison with Past Warnings
| FDA Warning (CAR T-cell Therapy) | Previous Warning (e.g., Chemotherapy Agent) | Key Similarities | Key Differences |
|---|---|---|---|
| Specific side effects, including cytokine release syndrome (CRS) and neurotoxicity. | Potential for severe side effects like peripheral neuropathy, myelosuppression, or organ damage. | Both highlight the importance of careful monitoring and potential for severe side effects. | CAR T-cell therapy warnings often focus on immune-related issues, while previous warnings might center on direct toxicity to specific organs. |
| Focus on long-term safety data. | Emphasis on identifying high-risk patients to minimize adverse events. | Both necessitate adjustments to treatment protocols. | CAR T-cell therapy emphasizes the complexity of the immune response, requiring a different approach to risk assessment. |
| Specific product-related issues. | Potential for specific drug interactions or drug-induced hypersensitivity reactions. | Both illustrate the need for careful consideration of potential adverse effects. | CAR T-cell therapy often involves genetic modification and complex cellular interactions, which are not present in the same way with traditional therapies. |
Public Understanding and Perception: Fda Cancer Car T Warning
The FDA’s recent warning regarding CAR T-cell therapy highlights a crucial aspect of modern medicine: balancing the potential benefits with inherent risks. Understanding how the public perceives this warning is paramount for effective patient support and ensuring informed decision-making. The complexity of the therapy, coupled with the emotional weight of a cancer diagnosis, necessitates clear and accessible communication.
A Simple Explanation for the General Public
The FDA warning, while not an outright ban, cautions against potential risks associated with CAR T-cell therapy. These therapies, designed to target and destroy cancer cells, can sometimes trigger adverse effects. The warning emphasizes the need for careful monitoring and management of these potential complications, ensuring that patients are well-informed and supported throughout their treatment journey. It’s essential to distinguish between the potential risks and the overall effectiveness of the therapy.
Potential Public Concerns and Anxieties
Patients and families facing cancer diagnoses often experience heightened anxiety. The FDA warning may exacerbate these concerns, leading to apprehension about the safety of the treatment. Fear of the unknown and uncertainty surrounding the long-term effects of CAR T-cell therapy are legitimate anxieties. This is further compounded by the potential for severe side effects, which can range from manageable discomfort to life-threatening complications.
Patient and Family Interpretations
Patients and their families might interpret the FDA warning in various ways. Some may view it as a reason to reconsider CAR T-cell therapy, fearing potential harm. Others might see it as a necessary precaution, recognizing the importance of careful monitoring and potential long-term consequences. The emotional impact of a cancer diagnosis can significantly influence how individuals process information about the therapy and the warning.
A patient undergoing treatment might feel vulnerable and uncertain, needing additional reassurance from healthcare professionals.
The FDA’s recent warning about potential risks with CAR T-cell therapy for cancer is definitely concerning. It’s a sobering reminder of the complex challenges in cancer treatment. Thinking about the resilience of Holocaust survivors, like those beautifully captured in Gillian Laub’s portraits holocaust survivor portraits gillian laub , makes me reflect on the human spirit’s capacity to overcome adversity.
Ultimately, the FDA’s work to ensure patient safety in cancer treatments is crucial.
Clear Communication Between Healthcare Providers and Patients
Open and honest communication between healthcare providers and patients is critical. Providers must explain the warning in a way that is easy to understand, addressing both the potential benefits and risks of CAR T-cell therapy. Active listening, acknowledging patient anxieties, and providing clear, actionable steps to mitigate potential complications are key elements of this communication. Providing educational resources and support groups can also be vital in alleviating anxieties.
Resources for Patients
The importance of providing readily available information to patients cannot be overstated. This section details resources designed to facilitate informed decision-making.
- FDA website: The FDA website is a primary source of information about the warning, providing details about the specific risks and recommended safety measures.
- Cancer support organizations: Organizations like the American Cancer Society and the National Cancer Institute offer valuable information, support groups, and resources for patients and their families. These resources provide a wealth of knowledge and support.
- Healthcare provider: Patients should always consult with their healthcare provider to discuss the FDA warning and its implications for their individual treatment plan. Their input is crucial in tailoring the approach to the patient’s specific circumstances.
- Online forums and support groups: Sharing experiences and insights with other patients and their families can offer valuable perspectives and support. These online platforms can provide emotional and informational support for those facing similar situations.
Safety Monitoring and Reporting Procedures
The FDA’s commitment to patient safety is paramount in the development and use of CAR T-cell therapies. Robust safety monitoring and reporting procedures are crucial to identifying potential risks and adapting treatment protocols as needed. This ensures that these innovative therapies are used safely and effectively, minimizing adverse events while maximizing benefits.The FDA employs a multi-faceted approach to monitor the safety of CAR T-cell therapies.
This involves continuous data collection, analysis, and evaluation of information from various sources, including clinical trials, post-market surveillance, and patient reporting systems.
FDA’s Monitoring Procedures
The FDA meticulously monitors the safety of CAR T-cell therapies through rigorous data collection and analysis. This involves reviewing data from ongoing clinical trials, tracking adverse events in patients receiving the therapy, and collaborating with researchers and healthcare providers. The agency’s commitment extends beyond the initial clinical trial phase, encompassing post-market surveillance to detect potential long-term effects. These comprehensive safety measures ensure that the treatment’s benefits outweigh any associated risks.
Adverse Event Reporting Mechanisms
Effective reporting mechanisms are essential for promptly identifying and addressing adverse events. Patients, healthcare providers, and researchers play vital roles in reporting any suspected side effects associated with CAR T-cell therapies. The FDA relies on these reports to understand the full spectrum of potential complications and adjust treatment protocols accordingly.
Updating Information and Guidelines
The FDA actively updates its guidelines and information based on new data. The agency’s commitment to safety involves adapting to evolving scientific understanding and incorporating insights from ongoing research. This adaptability is crucial for maintaining the safety and efficacy of these innovative therapies. The continuous review process enables the FDA to provide the most up-to-date and reliable information to healthcare providers and patients.
Patient Reporting Options
Patients play a critical role in reporting adverse events. The FDA provides various avenues for patients to report side effects, ensuring their voices are heard and their experiences are documented. By utilizing these reporting mechanisms, patients can contribute to the ongoing safety monitoring of CAR T-cell therapies.
| Reporting Method | Description | Contact Information (Example) | Further Information |
|---|---|---|---|
| Online Portal | A user-friendly platform for submitting reports electronically. | FDA website (link to be provided by FDA) | Detailed instructions and forms for reporting. |
| Phone Number | A dedicated hotline for reporting adverse events. | 1-800-FDA-INFO | Provides immediate assistance and support. |
| Mail Address | Physical address for submitting written reports. | Food and Drug Administration, 10903 New Hampshire Ave, Silver Spring, MD 20993 | Required format and details for written reports. |
| Healthcare Provider | Reporting through a healthcare professional. | N/A | Healthcare providers are critical in reporting adverse events, especially in a timely manner. |
Comparison with Other Cancer Therapies
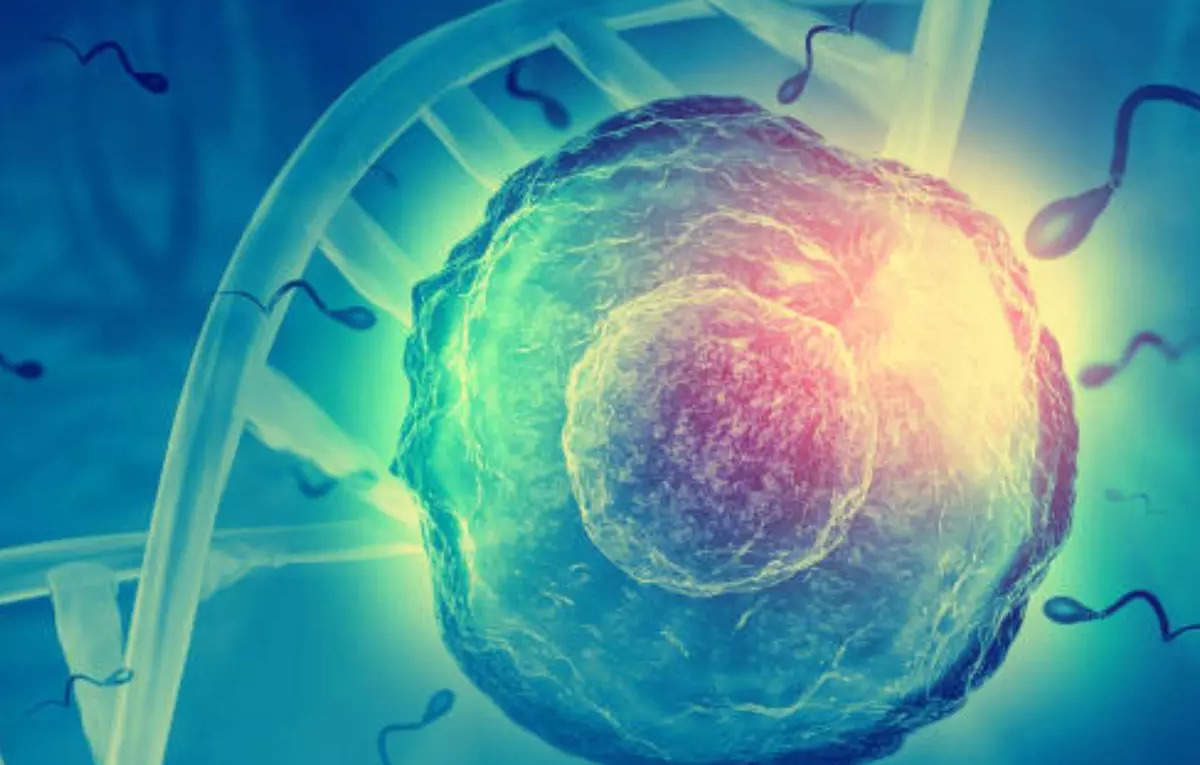
CAR T-cell therapy, while revolutionary, isn’t the only tool in the cancer arsenal. Understanding its place alongside established treatments is crucial for informed decision-making. This section delves into the safety profiles, unique challenges, and potential risks and benefits of various cancer therapies, including CAR T-cell therapy, to provide a broader perspective on the landscape of cancer treatment.Traditional cancer treatments, like chemotherapy and radiation therapy, have been staples in the fight against the disease for decades.
However, CAR T-cell therapy represents a paradigm shift, offering a targeted approach that harnesses the body’s own immune system. This approach, while promising, comes with its own set of considerations.
Safety Profiles of Different Therapies
Various cancer treatments have different safety profiles, impacting patients’ well-being and quality of life. Chemotherapy, for example, can cause significant side effects like nausea, hair loss, and fatigue. Radiation therapy can lead to localized tissue damage and long-term complications. The side effect profiles of CAR T-cell therapy are distinct, presenting unique challenges.
Unique Challenges in Monitoring CAR T-cell Therapy Side Effects
CAR T-cell therapy’s unique mechanism necessitates specialized monitoring and management of side effects. Cytokine release syndrome (CRS) and neurologic toxicities are common, requiring careful attention to patient response and immediate intervention. The unpredictable nature of these side effects demands constant vigilance and adaptability in treatment protocols. Furthermore, the potential for long-term side effects necessitates long-term follow-up and research.
Potential Risks and Benefits of Different Therapies
Each cancer therapy carries a range of risks and benefits. Traditional treatments like chemotherapy and radiation offer proven efficacy against a broad spectrum of cancers but can be associated with significant side effects. CAR T-cell therapy, while offering the potential for durable responses in certain blood cancers, comes with a higher risk of severe, potentially life-threatening, side effects.
The decision of which therapy to pursue is a complex one, involving careful consideration of the individual patient’s cancer type, stage, overall health, and preferences.
Comparative Analysis of Cancer Therapies
| Therapy Type | Side Effect Profile | Success Rate | Cost |
|---|---|---|---|
| Chemotherapy | Significant, often debilitating side effects (nausea, hair loss, fatigue, etc.). | Variable, dependent on cancer type and stage; often effective in combination with other therapies. | Generally moderate to high, depending on the specific regimen. |
| Radiation Therapy | Localized tissue damage and potential long-term complications. | Effective in localized cancers, often used in conjunction with surgery or chemotherapy. | Moderate to high, depending on the complexity of the treatment. |
| Targeted Therapies | Generally less severe side effects than chemotherapy, but specific to the target. | Variable, dependent on the specific cancer type and the targeted pathway. | Generally high, due to the complexity of development and production. |
| CAR T-cell Therapy | High risk of severe side effects, including cytokine release syndrome (CRS) and neurotoxicity. Requires specialized monitoring. | High success rates in certain hematological malignancies, but not universally effective. | Extremely high, due to the specialized manufacturing and administration process. |
This table provides a simplified comparison; the nuances of each therapy are substantial and should be discussed with a healthcare professional. Success rates are highly dependent on factors such as the patient’s overall health and the specific type of cancer being treated. Costs vary significantly based on factors like treatment duration and the complexity of the therapy.
Future Research Directions
CAR T-cell therapy has shown remarkable promise in treating certain cancers, but significant challenges remain, particularly concerning long-term safety and efficacy. Future research must address these concerns and explore innovative strategies to optimize the benefits of this revolutionary approach. This exploration will require a multi-faceted approach, encompassing improved safety protocols, deeper understanding of the therapy’s mechanisms, and development of more precise targeting methods.The FDA warning highlights crucial areas needing investigation.
Understanding the long-term consequences of CAR T-cell therapy, including potential immune dysregulation and organ damage, is paramount. Innovative approaches to mitigate these risks and enhance the therapy’s effectiveness are crucial for its widespread clinical application. A deeper understanding of the intricate interplay between the immune system and CAR T-cells is essential to unlocking the full potential of this treatment.
Long-Term Effects of CAR T-Cell Therapy
The long-term effects of CAR T-cell therapy are a critical area of research. Studies must rigorously investigate the potential for chronic immune dysregulation, organ damage, and other adverse events that manifest over time. A detailed understanding of the underlying mechanisms driving these complications is essential for developing preventative strategies and targeted interventions. This research should encompass a comprehensive analysis of various patient populations, considering factors like age, pre-existing conditions, and the specific type of cancer being treated.
Innovative Approaches to Address Safety Concerns
Developing strategies to mitigate the risks associated with CAR T-cell therapy is a critical research priority. One promising avenue is exploring ways to engineer CAR T-cells with enhanced safety profiles. This might involve modifying the CAR construct to reduce off-target effects, or developing methods to control the duration of CAR T-cell activity. Another promising approach is the development of strategies to improve immune tolerance and prevent chronic inflammation.
These could include the use of immunomodulatory drugs or the development of novel cell therapies that help to restore immune balance. For example, therapies focusing on mitigating cytokine release syndrome (CRS) or neurologic toxicity are urgently needed.
Developing Safer and More Effective CAR T-Cell Therapies
Improving the safety and efficacy of CAR T-cell therapies requires a multifaceted approach. One potential avenue involves enhancing the specificity of CAR T-cell targeting. Precise targeting of cancer cells minimizes damage to healthy tissues and reduces the risk of off-target effects. Furthermore, developing methods for more controlled and sustained release of CAR T-cells could improve therapeutic efficacy while minimizing the risk of adverse events.
Innovative delivery methods, such as nanocarriers, could be explored to improve the targeting and efficacy of CAR T-cells.
Potential Research Project: Long-Term Effects of CAR T-Cell Therapy
This research project aims to investigate the long-term effects of CAR T-cell therapy in a cohort of patients treated for various cancers. The study will track patients for at least five years post-treatment, monitoring for immune-related adverse events, organ damage, and any other long-term complications. The research will employ a comprehensive approach, including detailed clinical assessments, blood tests, imaging studies, and immunological analyses.
A specific focus will be placed on identifying biomarkers that can predict the risk of long-term complications and guide the development of preventive strategies. The research team will also collaborate with experts in immunology, oncology, and bioinformatics to develop predictive models that could help in risk stratification and personalized treatment strategies. Data collected will be analyzed to identify patterns and correlations between specific CAR T-cell constructs, patient characteristics, and the development of long-term adverse effects.
Closure
In conclusion, the FDA Cancer CAR T warning necessitates a comprehensive approach to cancer treatment, including careful patient selection, ongoing safety monitoring, and potential alternative therapies. The future of CAR T-cell therapy depends on careful consideration of both its potential and its risks. This warning serves as a crucial reminder that advancements in medicine must be approached with rigorous scrutiny and a commitment to patient safety.
FAQ Section
What specific types of cancer are affected by the FDA warning?
The FDA warning specifically targets certain blood cancers, including lymphomas and leukemias, but further research is needed to determine the precise scope of affected cancers.
What are some alternative cancer treatments for patients impacted by the warning?
Alternative treatments may include chemotherapy, radiation therapy, targeted therapies, and other immunotherapies, depending on the specific cancer type and patient characteristics.
How can patients report side effects to the FDA?
Patients can report adverse events to the FDA via various channels, including online portals, phone numbers, and mail addresses. Specific contact information is available on the FDA website.
What are the key milestones in the history of CAR T-cell therapy?
Key milestones include early clinical trials, significant advancements in technology, and a growing understanding of the efficacy and safety profiles of different generations of CAR T-cell therapies.

